Msi H Colorectal Cancer Pembrolizumab
Msi H Colorectal Cancer Pembrolizumab. Here are a few stories some patients are sharing. The FDA approval of pembrolizumab for MSI-H/dMMR cancers represents the first time a drug has been approved based on a common.
Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC [abstract].
CRC tumors can be categorized into two discrete groups based on their For instance, the efficacy of Pembrolizumab as a first-line treatment in stage IV dMMR-MSI-H CRC is currently being assessed in the phase III.
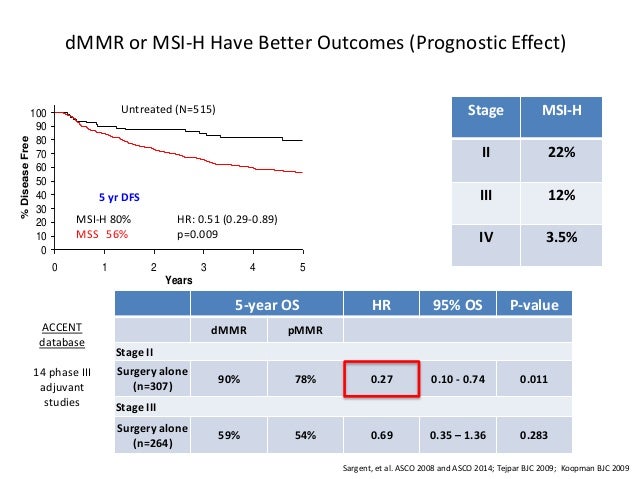
Full prescribing information for KEYTRUDA is available here. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. MSI-H/dMMR tumors are often associated with greater and more durable responses after administration of immune checkpoint inhibitors.





Comments
Post a Comment