Kras Mutation Colon Cancer
Kras Mutation Colon Cancer. The FDA approved therascreen KRAS RGQ PCR Kit for use in patients with metastatic colorectal cancer to determine KRAS mutation status. encoded search term (Colorectal Cancer and KRAS/BRAF) and Colorectal Cancer and. KRAS-mutation status in relation to colorectal cancer survival: The joint impact of correlated tumour markers.
KRAS mutation is linked to worse survival in liver metastases from primary colon cancers than for those liver metastases from rectal cancers.
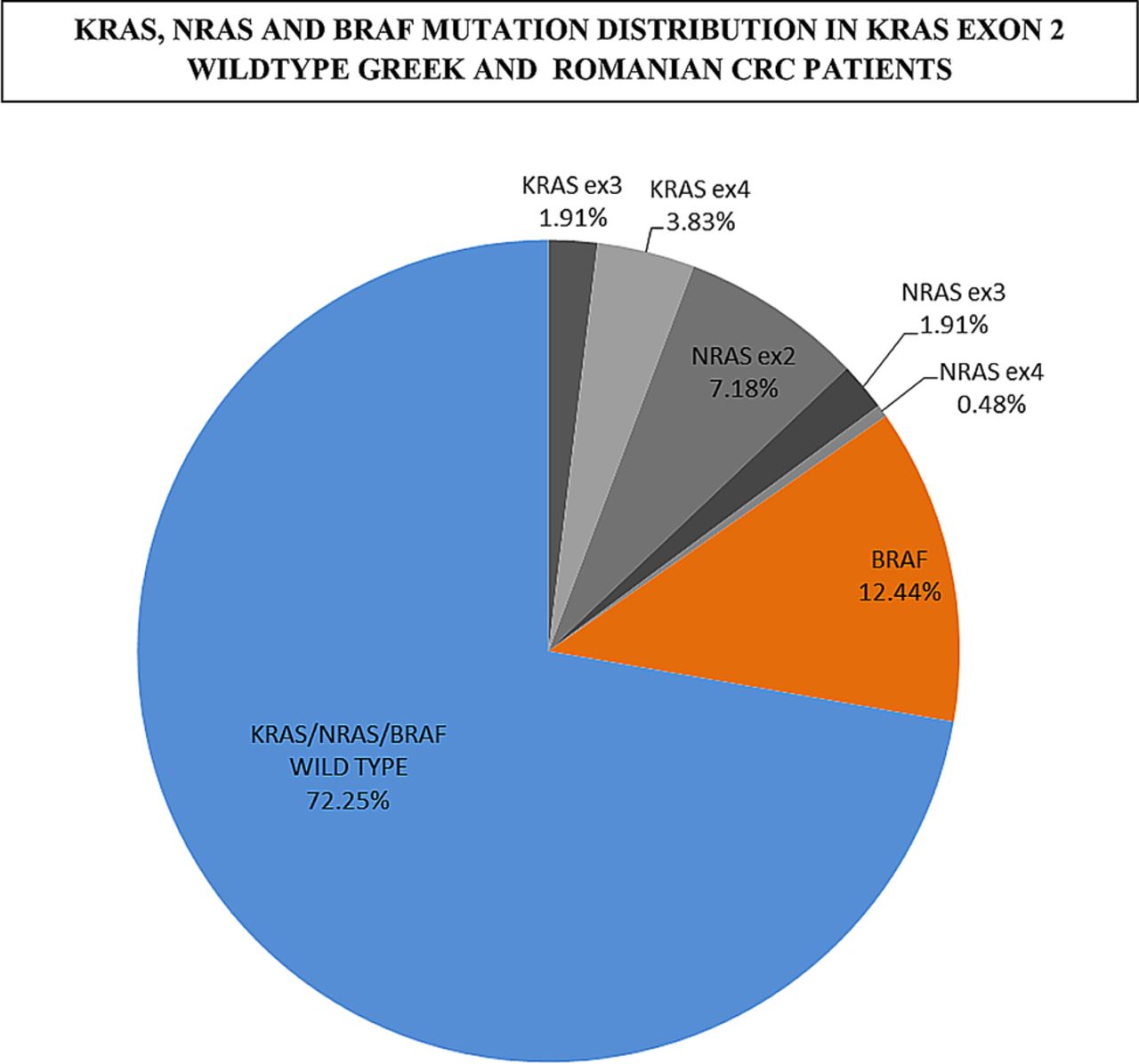
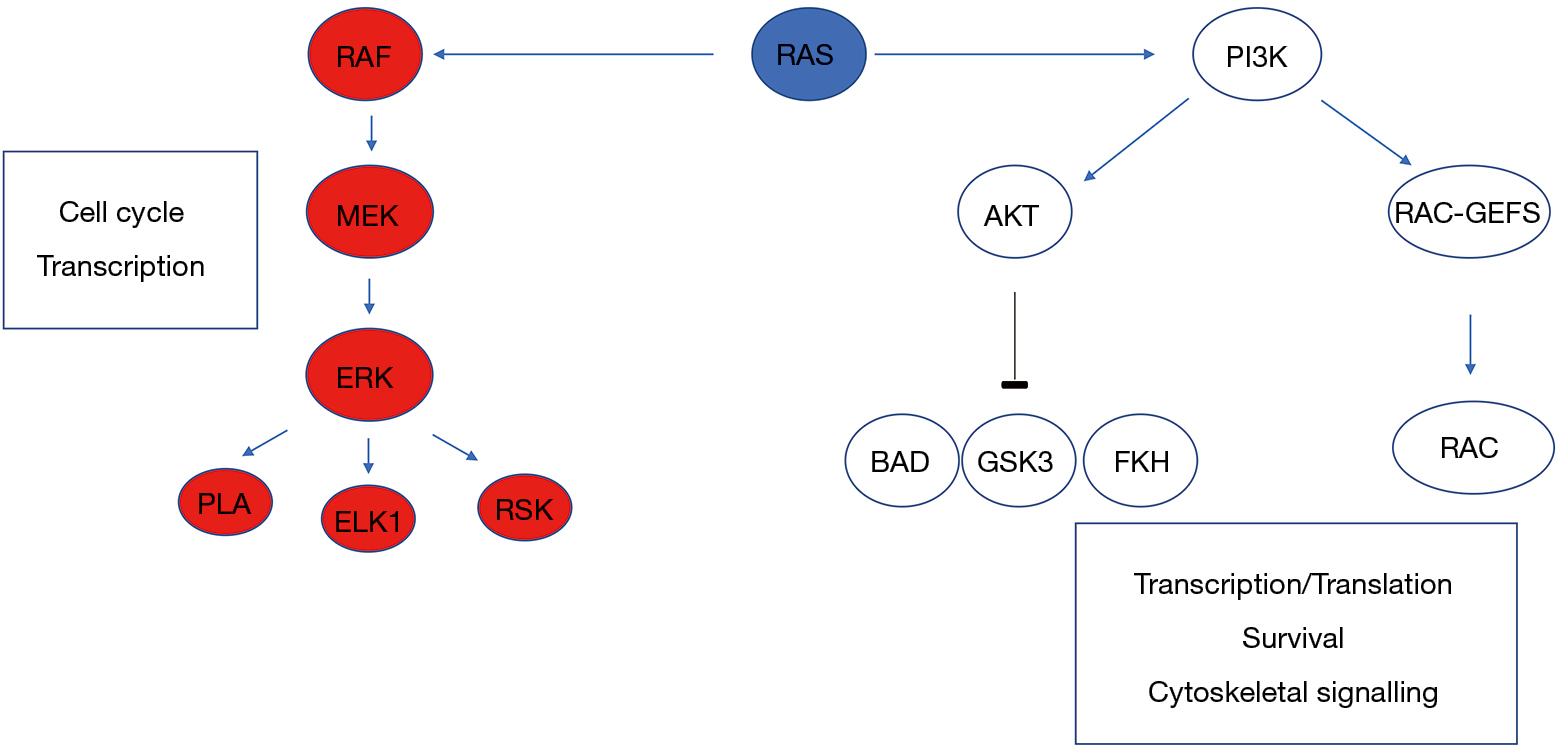
KRAS is the most frequently mutated, followed by NRAS and HRAS.
Activating mutation of the KRAS oncogene is an established negative predictor for anti-epidermal growth factor receptor (anti-EGFR) therapies in metastatic colorectal cancer (CRC). SAN FRANCISCO - When it comes to KRAS mutational status, liver metastases originating from left-sided colon tumors have different clinical and. ORLANDO-Unlike their wildtype counterparts, colorectal cancer tumors that harbor mutations in the KRAS gene do not respond to treatment with EGFR monoclonal antibodies.




Comments
Post a Comment